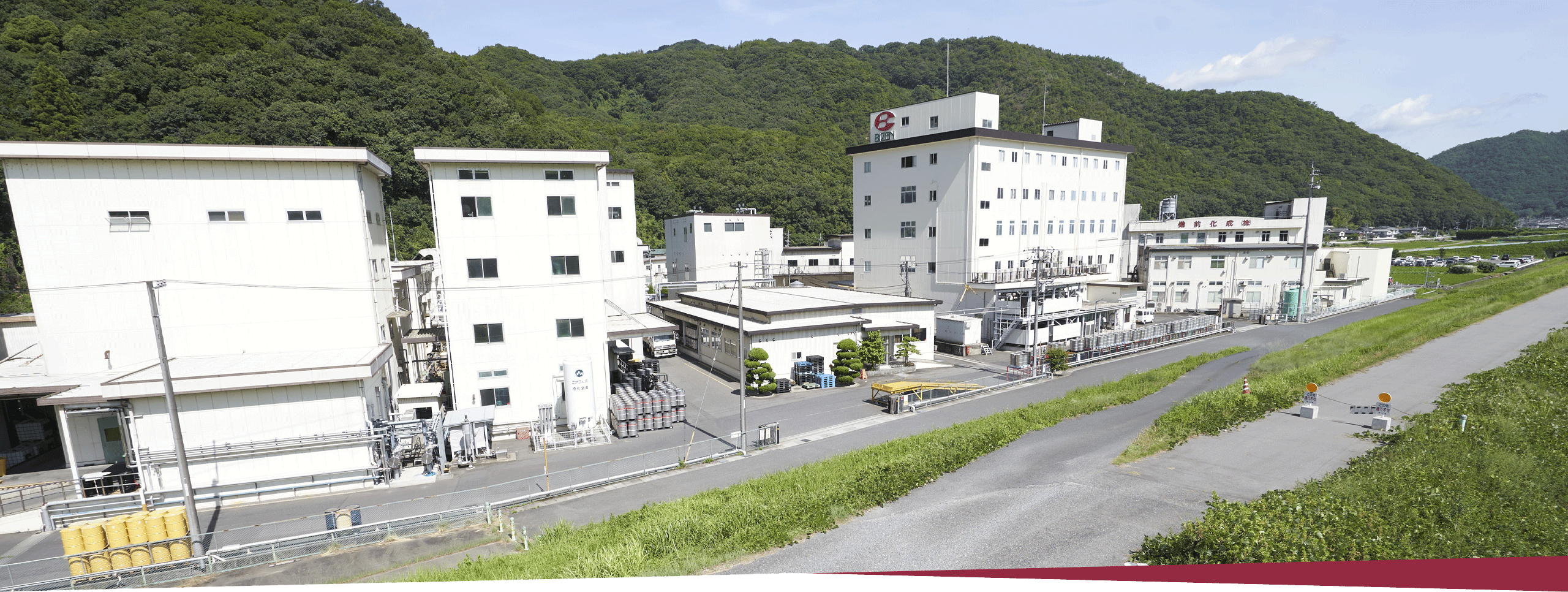
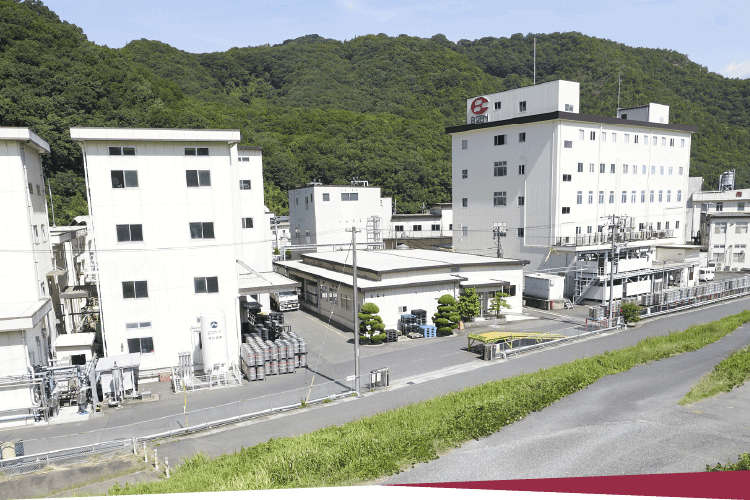
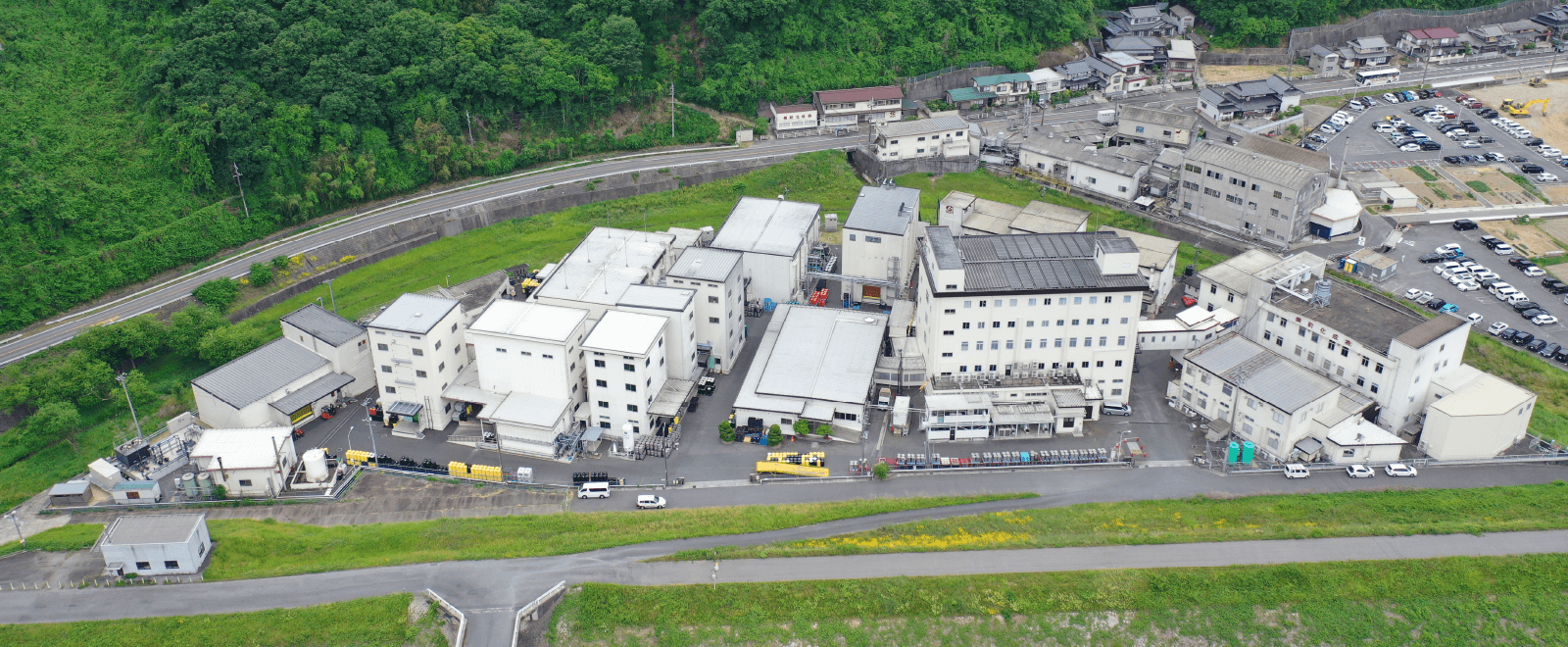
Company Outline
| Company Name | Bizen Chemical Co., Ltd. |
|---|---|
| Established | December 24, 1971 |
| Headquarters |
709-0716 363 Tokudomi, Akaiwa-shi, Okayama, Japan Telephone: +81-86-995-3311 (main) FAX: +81-86-995-3131 Google Map About 5 minutes by taxi from Mantomi Station on the Sanyo Main Line |
| Tokyo Office |
2-6-1 Nihonbashihoncho, Chuo-ku, Tokyo Google Map |
| Capital | 50 million yen |
| Board Members |
President Tomie Shimizu Executive Vice President Hideki Syouno Board Director Hisao Takisawa Board Director Masashi Otani Auditor Miyuki Syouno |
| Employees | 316 (as of Apr 1, 2025) |
| Primary Business |
Manufacture and sale of pharmaceuticals (APIs for ethical drugs, over-the-counter drugs), quasi-drugs, health food materials, and health food products |
| Acquisition of Certifications |
Health Food Products GMP  On November 9, 2005, we obtained Good Manufacturing Practice (GMP) certification for dietary supplements from the Japan Health and Nutrition Food Association. ISO 9001  As of July 18, 2006, the Japanese subsidiary of the British Standards Institution (BSI) certified that our quality management system is being operated effectively. FSSC 22000 
FSSC 748331/ On January 24, 2022, we obtained the FSSC 22000 international standard for food safety management systems certification from the Japanese subsidiary of the British Standards Institution (BSI). EU-HACCP As of March 1, 2011, we have obtained Ministry of Health, Labour and Welfare certification for facilities handling fish and fishery products to be exported to the EU. FDA GMP in API Manufacturing We have passed the GMP inspection for the manufacturing of API pharmaceutical intermediates by the US Food and Drug Administration (FDA). |
| Business Permits |
Type 2 pharmaceutical manufacturing and sales business 33A2X00012 Quasi-drug manufacturing and sales business 33D0X00008 Cosmetics manufacturing and sales business 33C0X00020 Pharmaceutical manufacturing business 33DZ006016 Quasi-drug manufacturing business 33DZ006016 Cosmetics manufacturing business 33CZ009024 Edible oleo manufacturing business (Bizen Public Health Center) No. 089100 Additive manufacturing business (Bizen Public Health Center) No. 330056 Confectionery manufacturing business (Bizen Public Health Center) No. 290131 Dairy manufacturing business (Bizen Public Health Center) No. 300184 |
| Affiliated Organizations | Japan Health and Nutrition Food Association Kansai Pharmaceutical Industries Association CRN Japan The SAC Association L8020 Council DHA/EPA Council Okayama Bioactive Research Society GOED (Global Organization for EPA and DHA Omega-3s)   |
| Main Banks |
Chugoku Bank, Wake Branch MUFG Bank, Okayama-Ekimae Branch Tomato Bank, Wake Branch |
| Main Business Partners |
Bitapol Co., Ltd. Kyowa Hakko Bio Co., Ltd. Koyo Mercantile Co., Ltd. Sunfco Ltd. Kongo Yakuhin Co., Ltd. Toppan Inc. PMC Corporation Co., Ltd. |